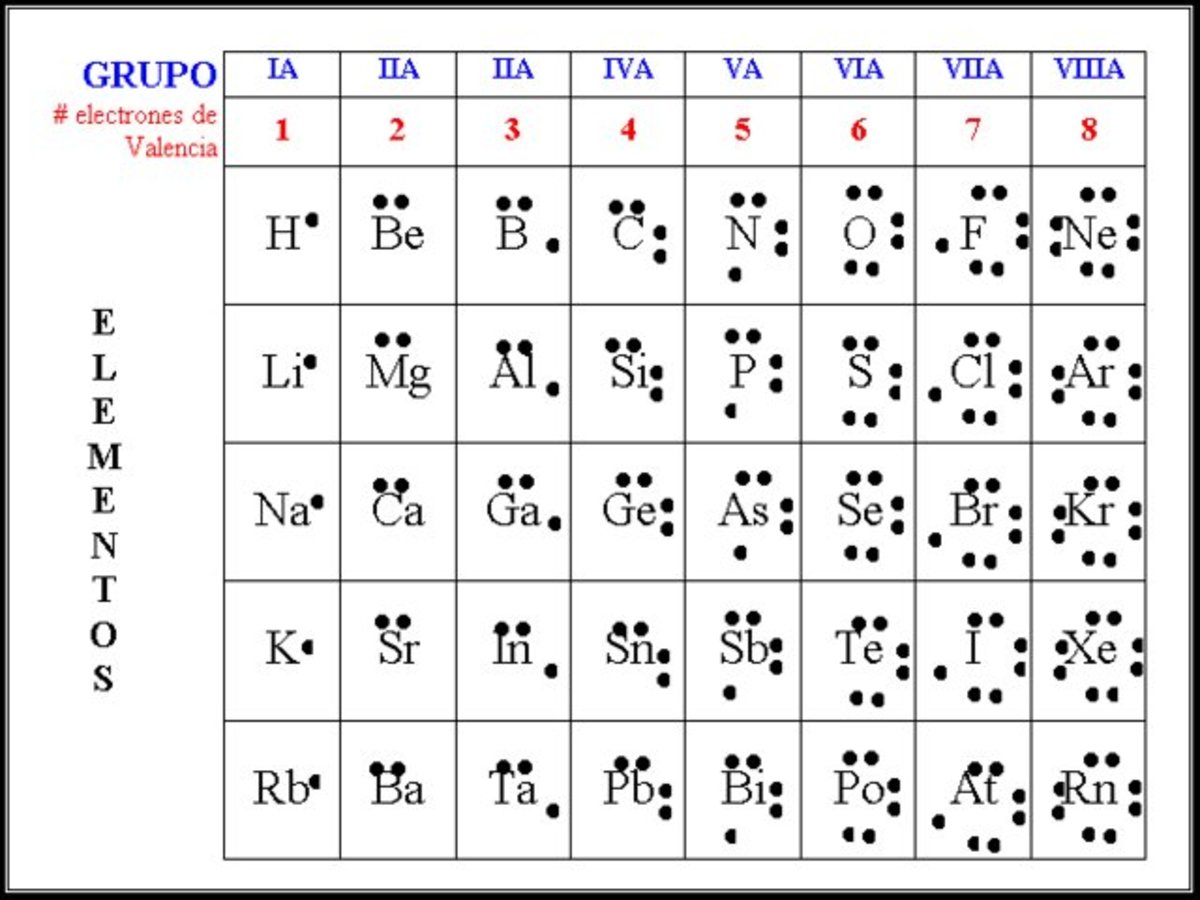
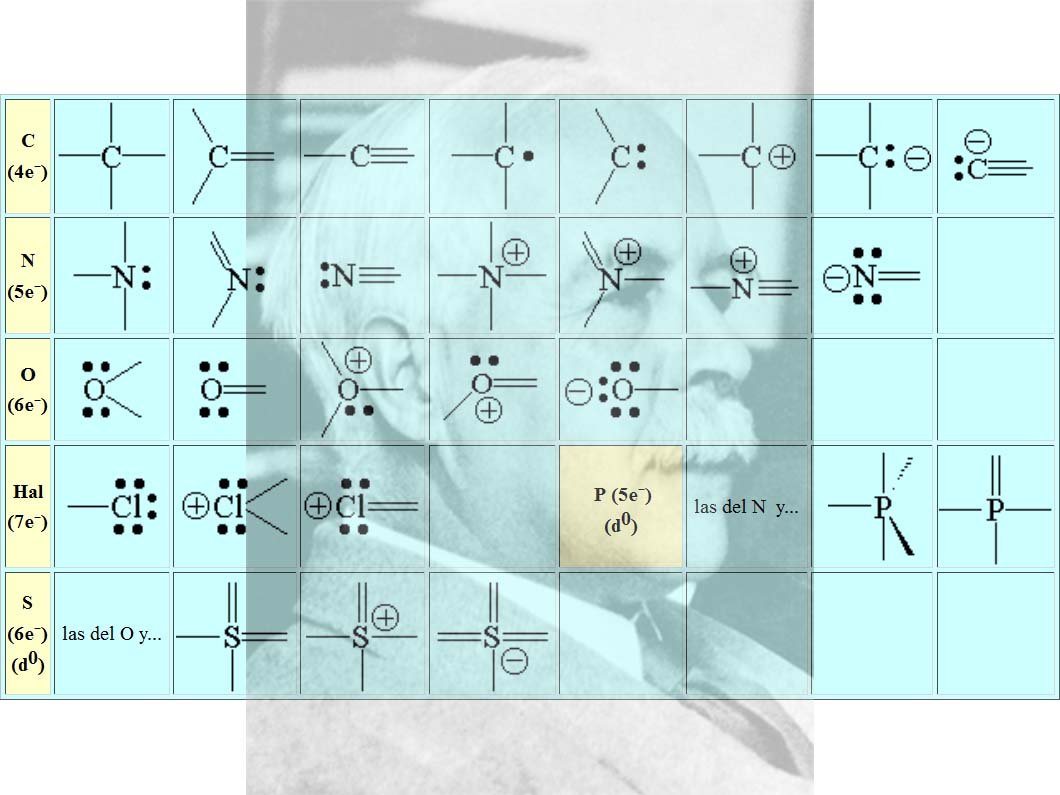
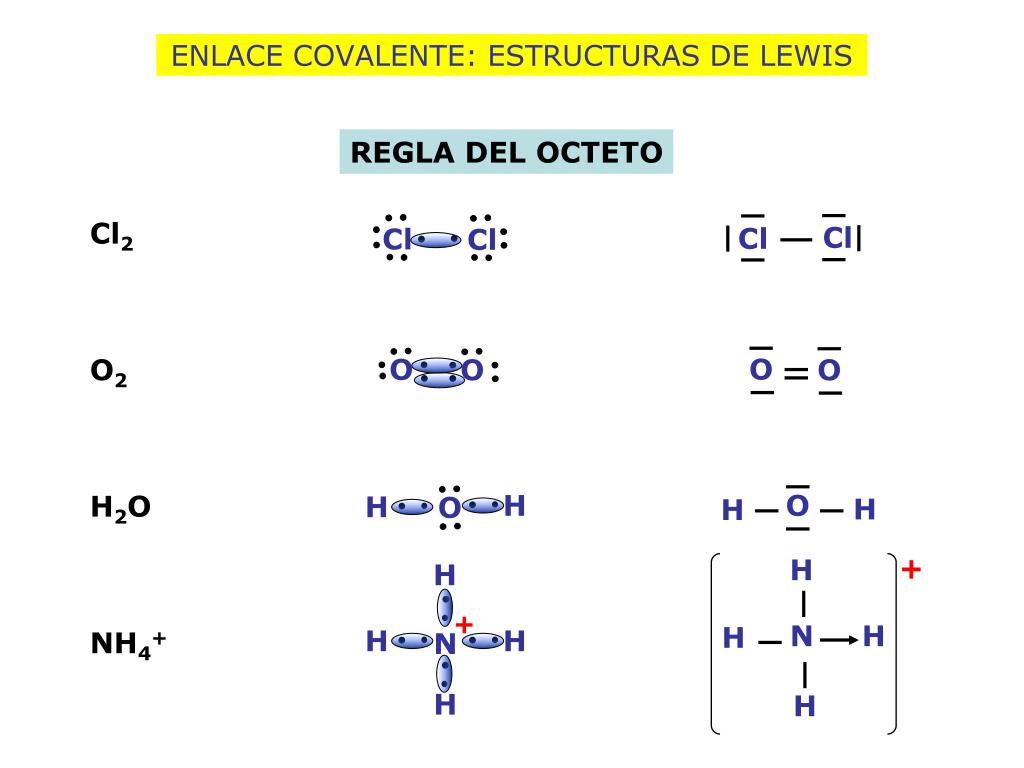
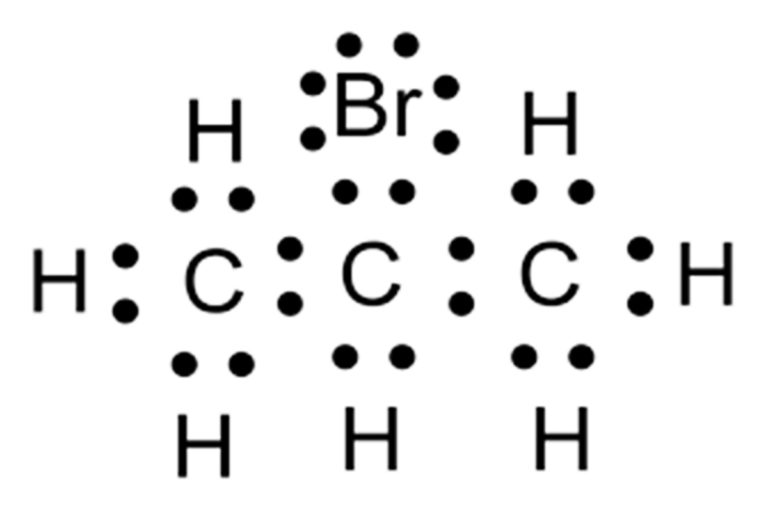
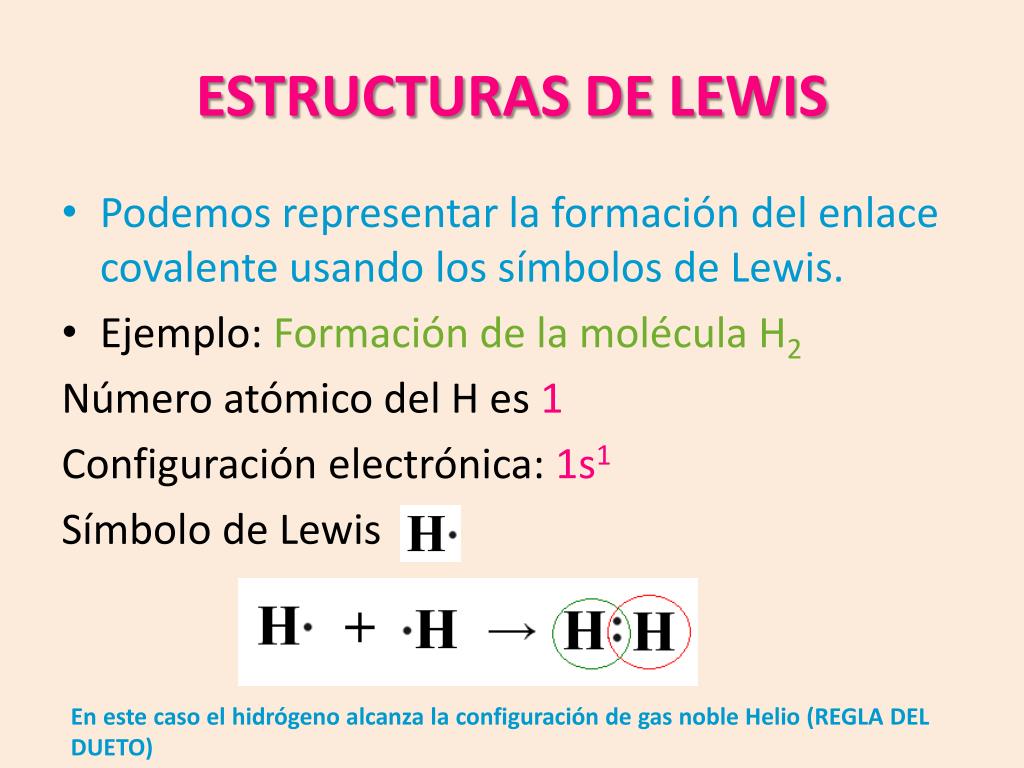
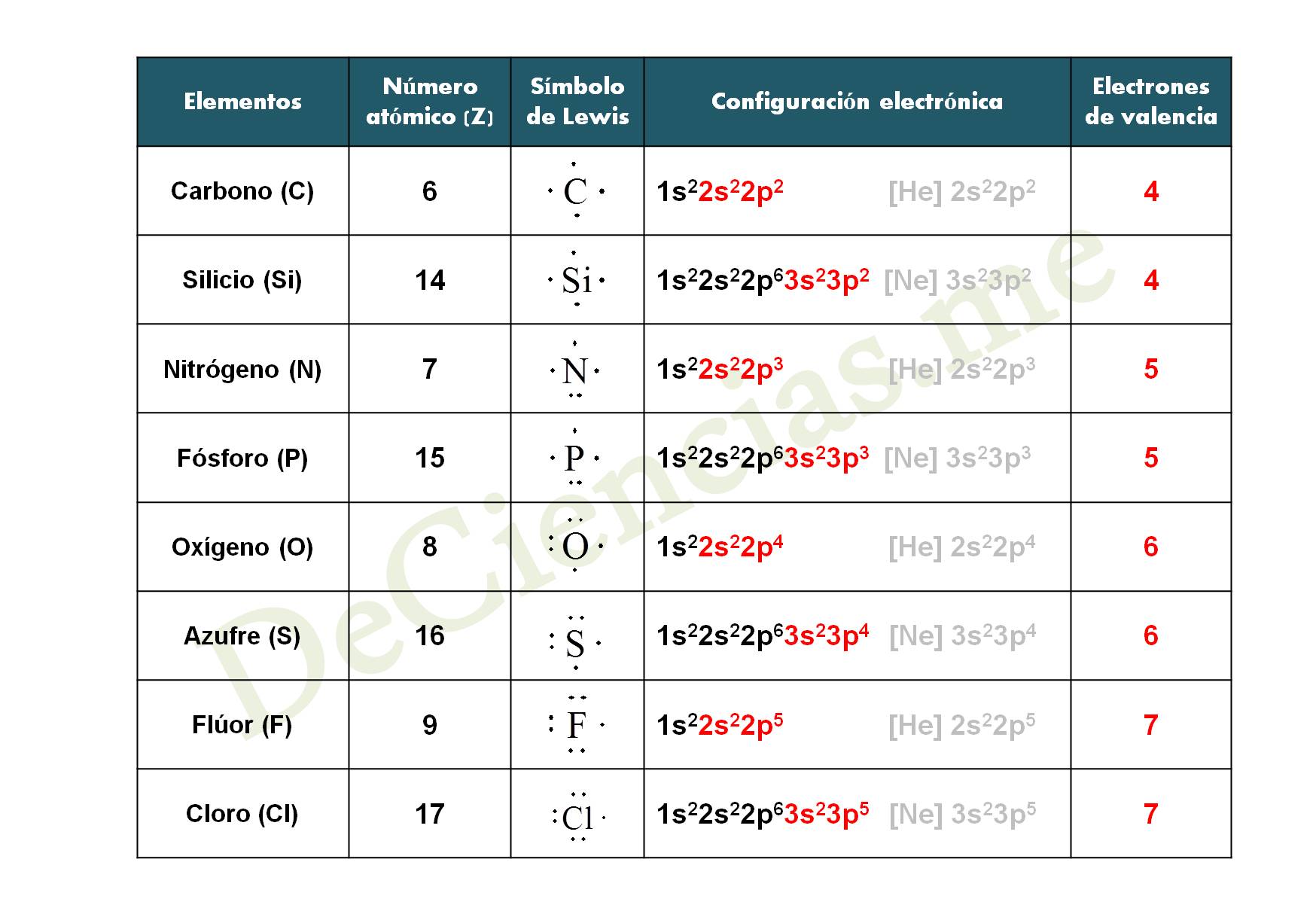
El diagrama de Lewis y Gilbert Newton Lewis
BeF2 crystallizes in the orthorhombic Cmmm space group. The structure is one-dimensional and consists of two BeF2 ribbons oriented in the (0, 0, 1) direction. Be2+ is bonded in a square co-planar geometry to four equivalent F1- atoms. All Be-F bond lengths are 1.62 Å. F1- is bonded in an L-shaped geometry to two equivalent Be2+ atoms.
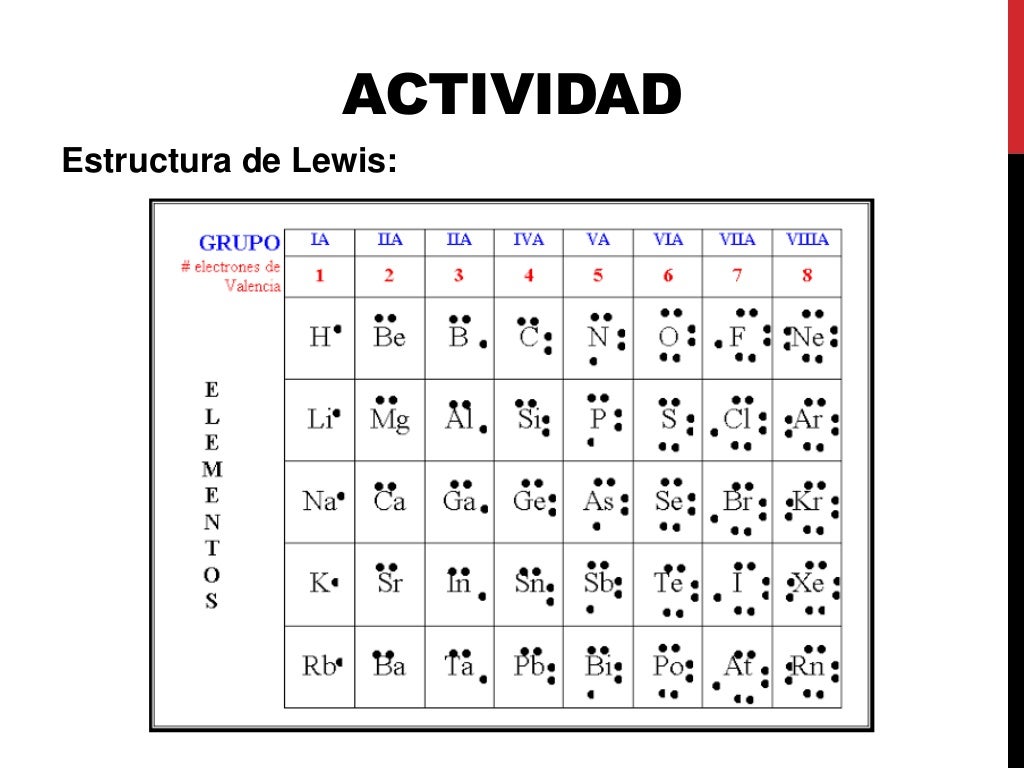
3 manières de dessiner une représentation de Lewis

A step-by-step explanation of how to draw the BeF2 Lewis Structure (Beryllium fluoride).In the Lewis dot structure for BeF2 beryllium only needs four valence.
Compartir electrones
For beryllium atom, formal charge = 2 - 0 - ½ (4) = 0. For each fluorine atom, formal charge = 7 - 6 - ½ (2) = 0. Here, both beryllium and fluorine atoms do not have charges, so no need to mark the charges. In the above structure, you can see that the central atom (beryllium) doesn't form an octet.
Estructura De Lewis
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
Qué es la estructura de Lewis Artículos de Arquitectura
BeF2 is quartz (beta)-like structured and crystallizes in the cubic I-43m space group. The structure is three-dimensional. Be2+ is bonded to four equivalent F1- atoms to form corner-sharing BeF4 tetrahedra. All Be-F bond lengths are 1.56 Å. F1- is bonded in a bent 150 degrees geometry to two equivalent Be2+ atoms.
Las estructuras de Lewis definición y características YuBrain

How can I draw the Lewis dot structure for BeF2? Chemistry Molecular Orbital Theory Molecular Geometry. 1 Answer Humaam H. Jul 10, 2014 Answer link. Related questions. How do I determine the bond angle in a molecule? Question #b4967 Question #2a64e.
Enlace químico. Tipos, características y ejemplos

70 More Lewis Dot Structures (***note Be is an exception and does not follow the octet rule) It will be constructed with 2 boning pairs, no lone pairs of electrons on the Be. All beryllium compounds are highly toxic. Beryllium fluoride is very soluble in water and is thus absorbed easily; it inhibits ATP uptake.
Lewis Structure of BeF2 [with video and free study guide]
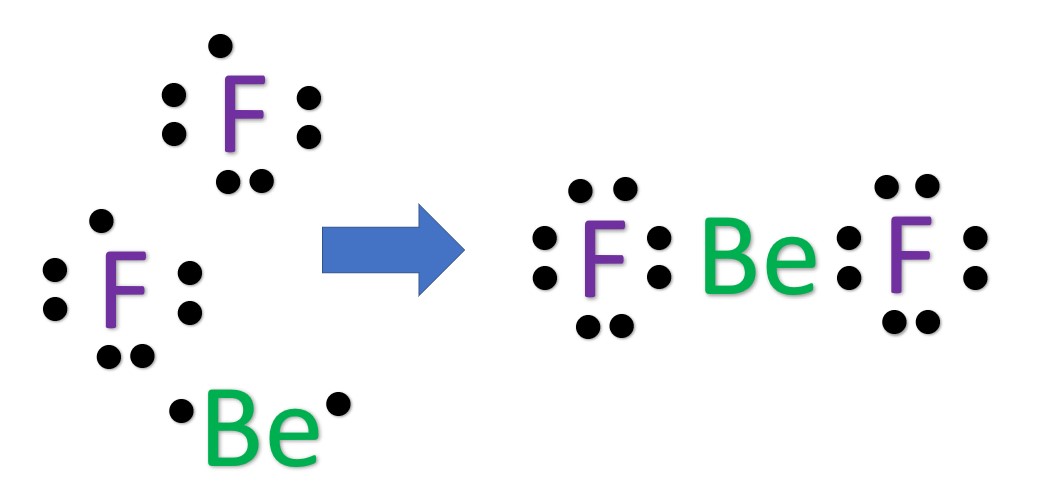
Step 2: Find octet electrons for each atom and add them together. Most atoms like 8 electrons to form an octet, but there are exceptions and Be is one of those exceptions, as it only needs four electrons for an octet. Be: 1×4 = 4. F: 2×8 = 16. Total = 20 "octet" electrons. Step 3: Find the number of bonding electrons.
Como Se Representa El Modelo De Lewis Noticias Modelo

Beryllium fluoride. Molecular Formula BeF. Average mass 47.009 Da. Monoisotopic mass 47.008987 Da. ChemSpider ID 22992. - Charge.
Qué es la estructura de Lewis Artículos de Arquitectura
I quickly take you through how to draw the Lewis Structure of BeF2, (beryllium fluoride). I also go over formal charge, hybridization, shape and bond angle.
Estructura De Lewis Imagenes
Another (easier) method to determine the Lewis structure of BeF 2: Alternatively a dot method can be used to draw the Lewis structure. Calculate the total valence electrons in the molecule. Be : 1×2 = 2. F : 2×7 = 14. Total = 16 valence electrons. Now, treat the atoms and electrons like puzzle pieces.
Tabla de estructura de lewis uDocz
this is the complete Lewis structure of CO 2. For Lewis structure purposes, the lone-pairs can only be moved from terminal atoms to the central atom to form multiple bonds, not the other way around. 7. Formal charges check: all atoms have formal charges equals to 0 in this structure. FC (C) = 4 -½× (4×2) = 0.
¿Qué son las Estructuras de Lewis? Diccionario de Química Orgánica Tu Blog de Ciencias
BeF2 is a chemical formula for Beryllium fluoride. It consists of one Beryllium and two Fluorine atoms. In this video, we help you determine its Lewis Struct.
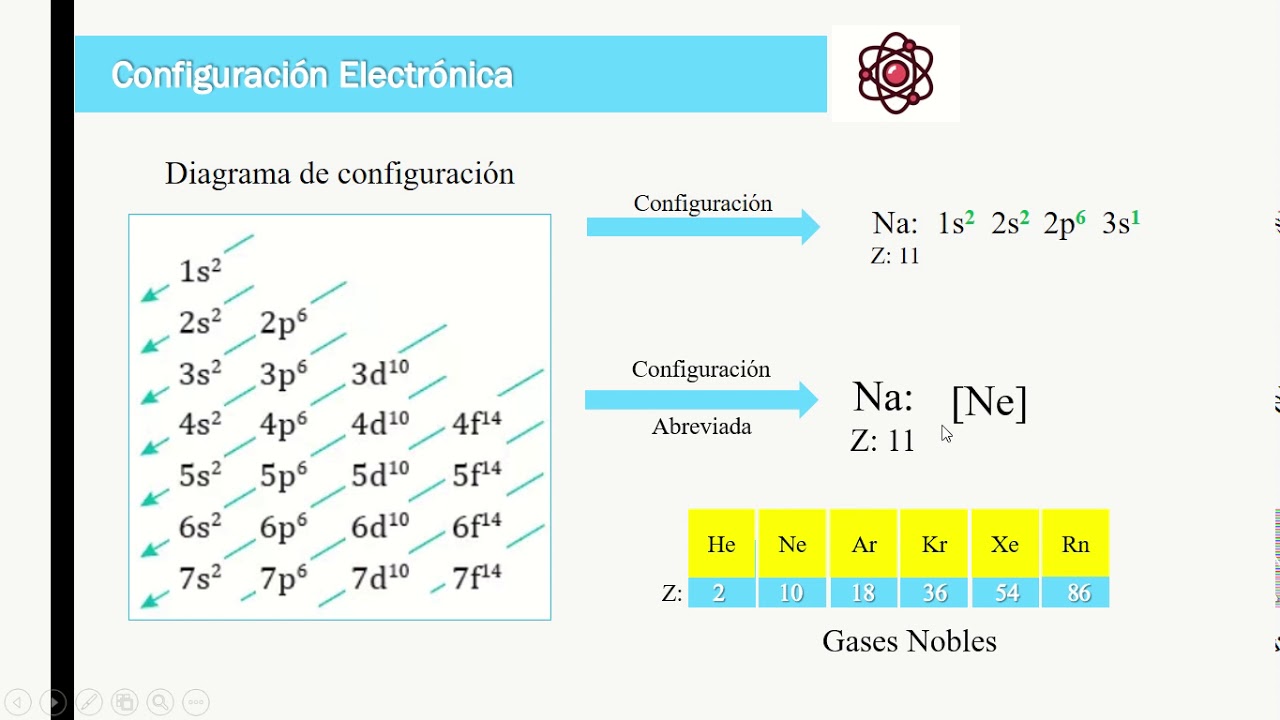
Aprende Configuración electrónica y simbología de Lewis YouTube
Step #1: Calculate the total number of valence electrons. Here, the given molecule is BeF2 (beryllium difluoride). In order to draw the lewis structure of BeF2, first of all you have to find the total number of valence electrons present in the BeF2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
Estructura de Lewis
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
BeCl2 Lewis Structure How to Draw the Lewis Structure for BeCl2 YouTube
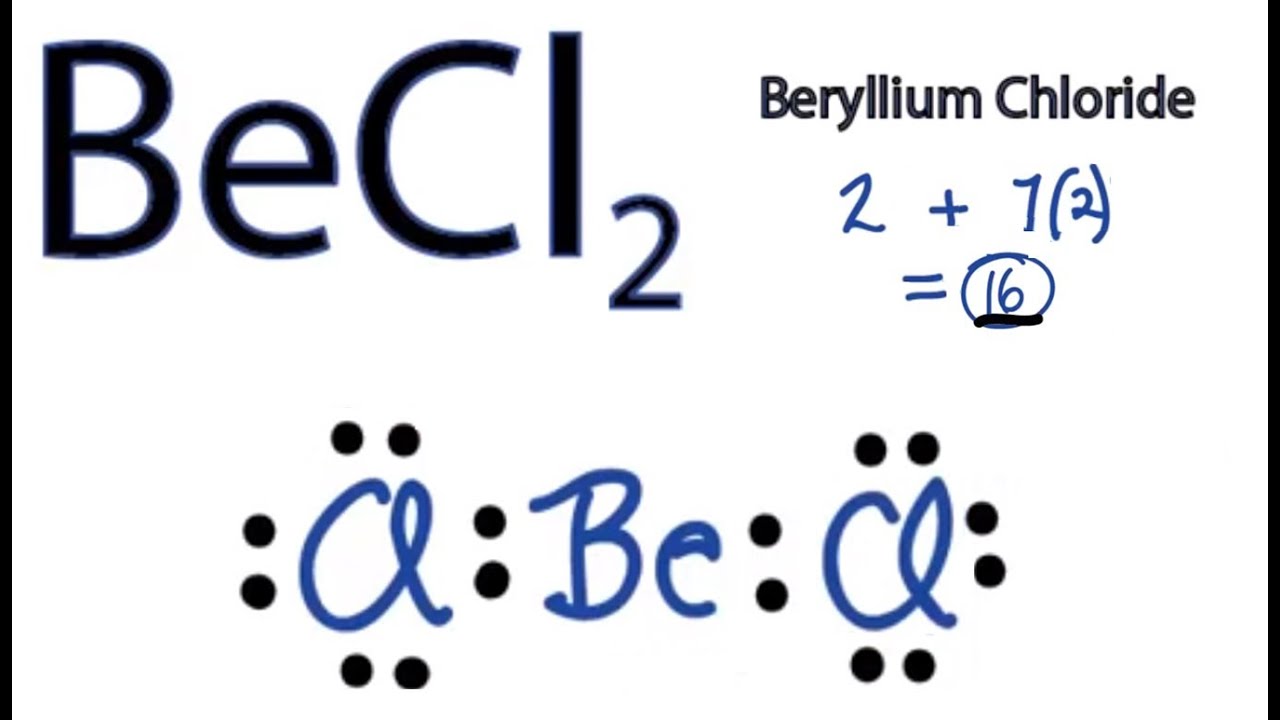
BeF2 crystallizes in the monoclinic C2 space group. The structure is two-dimensional and consists of one BeF2 sheet oriented in the (0, 0, 1) direction. there are two inequivalent Be2+ sites. In the first Be2+ site, Be2+ is bonded in a trigonal planar geometry to three F1- atoms. There are a spread of Be-F bond distances ranging from 1.41-1.54 Å.
.